Products
BioEngine has developed nearly 100 types of serum-free media for different cell lines, which are suitable for antibodies, vaccines and cell and gene therapy (CGT) fields.
Complete materials to ensure the provision of sample products within 2 weeks
The shortest delivery time of 1 month
Excellent batch-to-batch consistency
(RSD<10%;CPK>1.33)
Comprehensive formulation protection process and internal system to ensure information security
Up to 100,000 liters/batch of production and the stable supply of high-quality raw materials ensure more competitive product prices
Strictly selected "2+1" raw material supplier model allows customers to face the fluctuation of raw material market with ease
More than 80% of raw materials have more than 2 local suppliers and 1 foreign supplier
Be responsible for coordinating and providing technical services throughout the whole project.
Involved in full product life-cycle management from product design and process production to product application, and provide traceable data of product design and standards for audit.
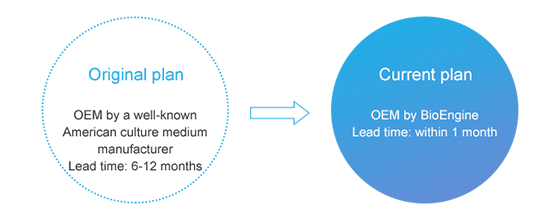
Help solve local supply demand and ensure the large-scale production progress of customers.
Dozens of batches of finished products have been delivered, and customers have given feedback that the product quality is stable with little fluctuation of trace element content (for example, the fluctuation of Mn element content at ppm level is less than 15%).
Customer evaluation: By comparison, the OEM capability of BioEngine reaches the international advanced level in terms of the trace element content.
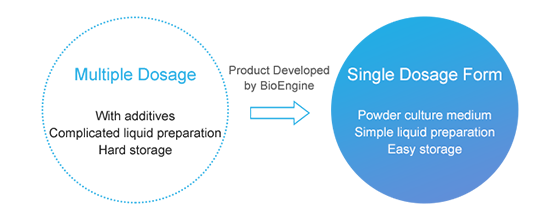
Help customer to upgrade the self-developed formulation and manufacturing to ensure final product is of high quality and has long-term stability same time.
Solve the degradation problem in the processing of raw materials with special properties in the original process, upgrade original "1+4" multi-dose solution to a single dose powder solution, reduce the costs of storage and transportation for customers and the difficulty of solution preparation.
Before receiving customer formulation, both sides need to enter into a confidentiality agreement before communicating with each other about the formulation. The legal person of Beijin Biotech, a wholly-owned subsidiary and supply chain center of BioEngine, is the sole recipient of the customer’s formulations. The received formulation will be evaluated in time and then transcoded and sent to OEM group of Product Development Department for further sample preparation. If the formulation or material needs to be adjusted, the formulation recipient will communicate directly with the customer, and the sampling process will be carried out once both parties confirm the new formulation.
According to the formulation analysis and raw material accounting, the sample lead time is about two weeks when all materials are available. If some materials are specified by customers, the delivery period will be projected according to the actual arrival date of the materials. The supply period for final products depends on the product testing period, generally ranging from two weeks to one month, due to the need for material preparation.
We have a professional technical team to provide high quality one-stop cell culture service from process development
and optimization, medium formulation design to medium processing and manufacturing for all biopharmaceutical companies.
If you have any question, please click "Quick Message" and leave your message, we will reply to you as soon as possible.
If anything urgent, please call (86)21-68582660-2792.
![]() 3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC
3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC