Products
BioEngine has developed nearly 100 types of serum-free media for different cell lines, which are suitable for antibodies, vaccines and cell and gene therapy (CGT) fields.
Time:May 05,2017
From April 1-6, 2017, Dr. Liang Zhao, Vice President of the company, Dr. Xuping Liu, Director of R&D, and Dr. Li Fan, led three Ph.D. students, Xinning Chen, Yixiao Wu, and Qian Ye from the Animal Cell and Tissue Engineering Research Laboratory, State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, to San Francisco, California, USA to attend the 253rd Annual Meeting of the American Chemical Society (ACS). The American Chemical Society (ACS) is a professional organization in the field of chemistry, founded in 1876, with 163,000 members from all branches of chemistry.
The ACS annual meeting is held twice a year, covering all fields of chemistry. The 253rd annual meeting of the ACS covers more than 40 fields, including analytical chemistry, glycol chemistry, biology, biochemical technology, physical chemistry, and organic chemistry. The biochemical technology session, in which six of us participated, was one of the largest sessions.

The picture showed the group photo of the technical team
On the morning of April 2, Dr. Qian Ye, a Ph.D. student, gave a unique oral presentation in the Biochemical Technology session, which was well received by the audience. The topic of her oral presentation was "Application of MDCK cells to establish an efficient serum-free influenza vaccine production process". The presentation explained the need for the development of serum-free suspension animal cells for influenza virus production in response to influenza outbreaks, and systematically and comprehensively described the establishment of an efficient influenza virus production process based on animal cells and the industrial application of this process in China from the aspects of cell line domestication, culture medium design, process optimization, and scale-up.

Pictured is Qian Ye giving an oral presentation in the biochemical technology session
In the afternoon of April 2, Dr. Liang Zhao shared his laboratory's research results on carbon dioxide in the biochemical technology session, entitled "The effect of carbon dioxide on cell metabolism in cell culture". He explained how high CO2 partial pressure affects cellular energy metabolism, lactate metabolism, and product expression from the cellular scale (metabolome), molecular scale (transcriptome), and reactor scale, and showed the metabolic flow of key metabolic pathways such as glycolysis and tricarboxylic acid cycle calculations. Experts and professors from many large pharmaceutical companies and universities came to discuss and exchange business cards with Dr. Liang Zhao, hoping to have further exchanges.

The picture showed Dr. Liang Zhao giving an oral presentation in the session on biochemical technology
On the evening of April 4, Dr. Xuping Liu, Dr. Li Fan, and Ph.D. students Xinning Chen and Yixiao Wu presented and explained the research results of the lab in the poster hall. Yixia Wu's poster was titled "Optimization of virus vaccine production process based on the high-density culture of animal cells". The poster mainly showed that the authors applied the serum-free medium developed by the lab, analyzed the difference in nutrient metabolism between virus-infected and non-infected groups, and finally established a simple and efficient nutrient flow addition process based on the end-process nutrient status, virus yield, virus single cell yield, virus infectivity, and morphology. During the meeting, Yixiao Wu communicated and presented with the participants from all walks of life, introducing them to the development situation of domestic viral vaccines and the establishment and application of this process in China, which was interesting to the listening attendees. Dr. Xuping Liu's poster was entitled "Uncovering the formation mechanism of antibody charge heterogeneity in CHO cell culture", which focused on the interconversion process of purified and collected antibody acidic variants, primary components, and basic variants by aseptic incubation in cell culture medium. Through chemical energy calculations as well as kinetic model calculations, the mechanism of the formation of charge heterogeneity of antibodies is revealed from another perspective and the formation of charge heterogeneity is linked to process parameters.
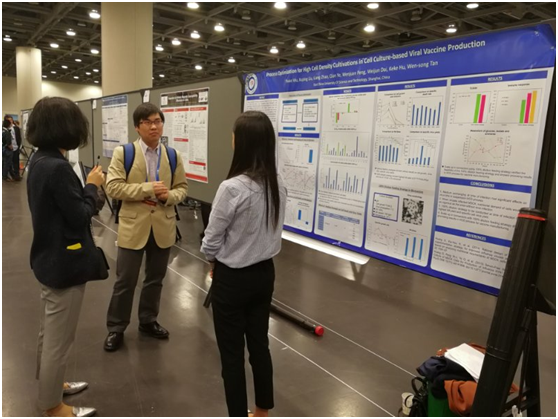
The picture showed Dr. Xuping Liu and Yixiao Wu communicating with the attendees
Dr. Li Fan's poster was titled "Regulation of high mannose glycoform ratio of therapeutic recombinant proteins by culture medium fraction". The poster by Xinning Chen was entitled "Regulation of sialylation of antibody fusion proteins in cell culture". Both of them are glycosylation studies. Dr. Li Fan showed how we use metabolomics and key gene expression analysis to reveal the key metabolic nodes and their metabolic markers in the glycosylation process, and through the addition and adjustment of associated nutrients to achieve a controlled cell culture process of monoclonal antibodies with high mannose glycoforms. Chen Xinning introduced to the participants that it is possible to calculate the proportion of salivary acidification problems generated during the production of the antibody fusion protein by adopting the derivation of intracellular and extracellular kinetics of cells, and also to understand the nature of salivary acidification problem generated by the analysis of key intracellular glycosylation processing nodes and the study of extracellular salivary acid degradation characteristics, and to establish the linkage with culture medium components and reactor parameters. He has successfully guided companies to implement a controlled cell culture process of salivation of antibody fusion proteins at an industrial scale.
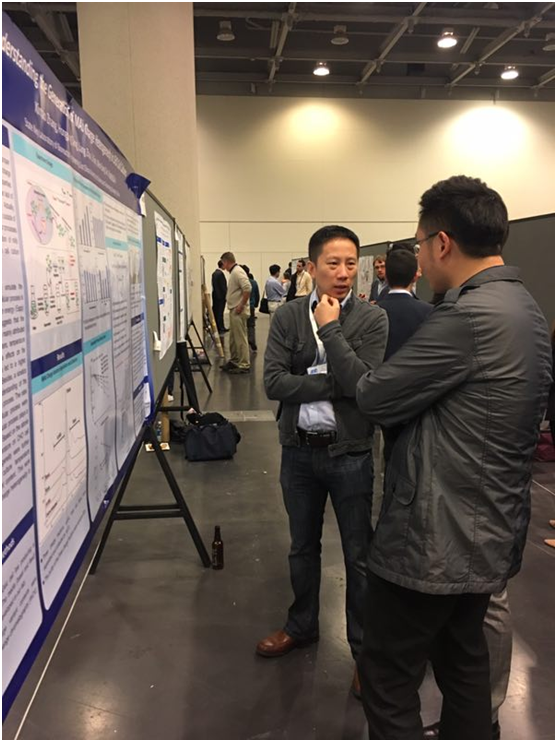
The picture showed Dr. Li Fan communicating with Dr. Anli Ouyang, Director of Lilly Biologics Research and Development
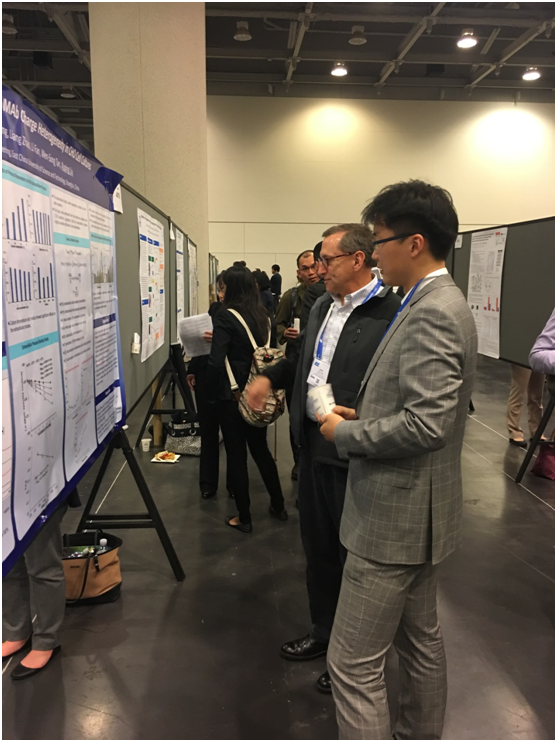
The picture showed Xinning Chen communicating with Dr. John T. Stults, Director of Protein Analysis at Genentech
During the poster session, we had lively discussions with industry experts from international pharmaceutical giants such as Genentech, Bristol-Myers Squibb, and Eli Lilly, as well as professors and scholars from renowned universities such as the University of Minnesota and the University of Delaware. Dr. Michael Borys, Director of Biologics Process Development at Bristol-Myers Squibb, said that product quality is given in advance through process design, which requires continuous in-depth process research to understand the process. He was very impressed with the process analysis ideas established in our lab and the kinetic calculation model to understand and control the process of biopharmaceutical manufacturing. Dr. John T. Stults, Director of Genentech's Protein Analysis Department, said that although they have been working on this for more than a decade, the mammalian cell system is so complex and the structure of biologics is so varied that they still cannot correlate the quality of the product with the parameters of the manufacturing process, so it is difficult to develop a truly controllable mammalian cell manufacturing process. He believes that only by continuously understanding the cells and exploring the formation mechanism of the problem as we do, we can finally do a good job in this difficult task of mammalian cell culture.
This time our technical team went to the US to attend the ACS annual meeting and achieved satisfactory results, not only to understand the new ideas and technologies of cell culture process control at the international level but also to successfully demonstrate the academic achievements and concepts of the technical team in the international arena. This team will also make further efforts to add bricks and bricks to the road to an international, strong, and prosperous future.
We have a professional technical team to provide high quality one-stop cell culture service from process development
and optimization, medium formulation design to medium processing and manufacturing for all biopharmaceutical companies.
If you have any question, please click "Quick Message" and leave your message, we will reply to you as soon as possible.
If anything urgent, please call (86)21-68582660-2792.
![]() 3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC
3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC